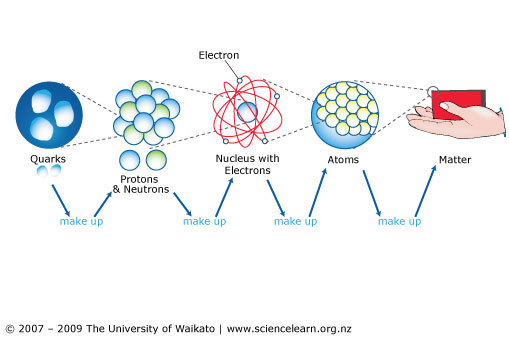
All matter has mass and occupies space. All physical objects are made of matter. Matter itself is composed of tiny building blocks known as 'atoms'. There are only 118 different types of atoms known to man. Frequently, atoms are bonded together to form 'molecules'. What is an atom Matter Physics FuseSchoolAtoms are tiny particles that are so small they are not possible to see with the naked eye, and are only barel.
The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons. Atoms fit together with other atoms to make up matter. Noun (history of science) A hypothetical particle posited by Greek philosophers as an ultimate and indivisible component of matter. (physics, chemistry) The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.
An atom is a specific type of matter of which larger constructs of matter are made. Matter itself is a very ambiguous concept; a cosmologist might consider everything that isn't radiation ( light etc) to be matter. On the other hand here's an exce.
Article- Atomic model
- Basic properties
- The electron
- The nucleus
- Development of atomic theory
- The beginnings of modern atomic theory
- Studies of the properties of atoms
- Models of atomic structure
- Advances in nuclear and subatomic physics
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. Electrons are attracted to any positive charge by their electric force; in an atom, electric forces bind the electrons to the nucleus.
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells.
This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. For additional information pertaining to nuclear structure and elementary particles, seesubatomic particles.
Atomic model
Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.
As noted in the introduction to this article, an atom consists largely of empty space. The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles. They can be created only with the addition of enormous amounts of energy, however, and are very short-lived.
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10−10 metre. The radius of an atom measures 1–2 Å. Compared with the overall size of the atom, the nucleus is even more minute. It is in the same proportion to the atom as a marble is to a football field. In volume the nucleus takes up only 10−14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring nuclear sizes is the femtometre (fm), which equals 10−15 metre. The diameter of a nucleus depends on the number of particles it contains and ranges from about 4 fm for a light nucleus such as carbon to 15 fm for a heavy nucleus such as lead. In spite of the small size of the nucleus, virtually all the mass of the atom is concentrated there. The protons are massive, positively charged particles, whereas the neutrons have no charge and are slightly more massive than the protons. The fact that nuclei can have anywhere from 1 to nearly 300 protons and neutrons accounts for their wide variation in mass. The lightest nucleus, that of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei are nearly 500,000 times more massive. Com mac.
Basic properties
Atomic number
The single most important characteristic of an atom is its atomic number (usually denoted by the letter Z), which is defined as the number of units of positive charge (protons) in the nucleus. For example, if an atom has a Z of 6, it is carbon, while a Z of 92 corresponds to uranium. A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance. Since it is the electrons that determine how one atom interacts with another, in the end it is the number of protons in the nucleus that determines the chemical properties of an atom.
- key people
- related topics
Contents
- Matter and it’s classification
- Mixture
- Atoms and Molecules
What is chemistry?
The branch of science which deals with the study of composition, structure, properties and change of matter is called chemistry.
Matter and it’s classification
Anything that occupies space and has weight and can be felt (by any one or more of our senses) is called matter.
Everything that is around us including the pen, book, pencil, air, all living beings are composed of matter. They all have mass and occupy space.
Classification of matter can be done by two ways – physical and chemical classification.
States of matter :
There are four states of matter in the universe: solid, liquid, gas and plasma. But, matter on earth exists mostly in three states: solid, liquid and gas.
Differences between solid, liquid and gas :
Generally, on heating – solid changes to liquid and liquid changes to gas. On the other hand, on cooling – gas changes to liquid and liquid changes to solid. Eg.
What is Plasma ?
At very high temperature(of stars), atoms lose their electrons. The mixture of electrons and nuclei forms plasma state of matter.
Like gases, plasma have not fixed shape and volume, and are less dense than solids or liquids. But unlike ordinary gases( which are neutral), plasmas are made up of electrically charged particles(ions and electrons). Plasma makes up the sun and other stars, and it is the most common state of matter in the universe as a whole.
Changes of Phase : freezing, melting, condensation, vaporization, sublimation and deposition
A phase is a distinctive form of a substance, and matter can change among the phases. It may take extreme temperature, pressure or energy, but all matter can be changed.
There are six distinct changes of phase which happens to different substances at different temperatures. The six changes are:
- Freezing – the substance changes from a liquid to a solid.
- Melting – the substance changes back from the solid to the liquid.
- Condensation – the substance changes from a gas to a liquid.
- Vaporization – the substance changes from a liquid to a gas.
- Sublimation – the substance changes directly from a solid to a gas without going through the liquid phase.
- Deposition: the substance changes directly from a gas to a solid without going through the liquid phase.
- Ionization – Ions are formed by gain or loss of electrons from an atom or molecule.
Elements
An element is a simplest form of a pure substance that can neither be decomposed into nor built up from simpler substances by ordinary physical or chemical process.
There are total 118 elements with their atomic number from 1 to 118. Out of which 92 ( approximation) occur naturally while the rest are prepared artificially in the laboratories.
Elements are further classified as metals, non- metals and metalloids. Out of the 118 elements of the periodic table, 84 are metals, 7 are metalloids and rests of them are non-metals.
1. Metals : Metals are generally solids (except mercury, which is the only metal which exists in liquid state in room temperature). They are good conductors of heat and electricity, and are malleable (they can be hammered into sheets) and ductile (they can be drawn into wire). Iron, Aluminum, Copper, Silver and Gold are common example of metal
2. Non-metals : Non-metals are generally found in gaseous state but iodine is found in solid state and bromine is the found in liquid state. They are usually poor conductor of heat and electricity and are not malleable and ductile. Examples of non-metal are carbon, hydrogen, oxygen, nitrogen, sulphur, etc.
3. Metalloids : The elements which possess both the characteristics of metals as well as non-metals are called metalloids. In their physical properties, they are more like the nonmetals, but under certain circumstances, several of them can be made to conduct electricity. Some examples of metalloid are Silicon, Arsenic, Bismuth, Antimony, etc.
Compound
A compound is a pure substance formed by the chemical union of two or more elements in a fixed proportional by weight. For example:- water is a compound of hydrogen and oxygen combined together in a fixed proportional 1:8 by weight.
The properties of compound are entirely different from it’s constituents. For example, hydrogen burns, oxygen supports burning but water(containing hydrogen and oxygen) neither burns nor supports the burning.
Atom And Matter Quiz
Mixture
Anything obtained by mixing two or more substances (elements or compounds) in any proportion so that their components do not lose their identity is called mixture. Mixture can be separated by different method depending upon the nature of mixing components. Some of the methods are filtration, sublimation, evaporation, distillation, crystallization, etc.
Mixture is of two types:-
1. Homogenous mixture:-
The mixture in which the components mixed are uniformly distributed throughout the mixture is called homogeneous mixture. The mixing components cannot be seen. Homogeneous mixtures are also called as solutions
Example: – alcohol in water, air, petrol, sugar solution, etc.
2. Heterogeneous mixture:- Macupdate.
The mixture in which the components mixed are not uniformly distributed in the mixture is called heterogeneous mixture. The mixing components can be seen through our naked. Examples:- Mixture of oil and water, sand and water, iron and wood dust, etc.
Differences between compound and mixture :
| Compound | Mixture |
|
|
Atoms and Molecules
Atom :
An atom is the smallest particle of an element which can take part in chemical reaction. Atom consists of three fundamental particles like proton, neutron and electron. Atoms of same elements are similar in properties whereas atoms of different elements are different in properties. Example:- ‘H’ represent the atom of hydrogen.
Atoms may or may not have independent existence. Atoms of inert gases like helium, neon, argon, etc. have independent existence whereas atoms of oxygen, nitrogen, etc. do not have independent existence. Atoms combine with each other to form stable molecules (like O2).
Molecules :
Molecule is the smallest unit of an element or a compound which can exist in free state in nature and possess all the properties of the element or compound.
Atom And Matter
→ Molecules containing atoms of same element are called homoatomic molecules. Eg. H2, N2, P4, O2, O3, S8, etc.
→ Molecules containing atoms of different elements are called heteroatomic molecules. Eg. HCl, CO2, NH3, CH4, PCl5, etc.
Depending on the number of atoms presenet, a molecule is called monoatomic, diatomic and polyatomic molecule. Eg.
- Monoatomic molecule – He, Ne, Ar, Kr, Xe and Rn.
- Diatomic molecule – H2, NaCl, O2, HCl, etc.
- Polyatomic molecule – P4, NH3, H2SO4, etc.
Difference between compound and molecule :
Atom And Matter Bethany Mota
- A compound is a substance that is composed from two or more different elements. Eg. Water (H2O), table salt (NaCl), carbon dioxide (CO2), methane (CH4), etc .
- A molecule is a group of two or more atoms held together by chemical bonds. Eg. H2 , NaCl, N2, etc.
- All compounds are molecules but all molecules are not compound. Things like nitrogen gas (N2), oxygen(O2), etc.are molecules but not compounds since they only contain one kind of element.
Objective questions
Q 1) Air is an example of :
a. compound.

b. homogeneous mixture.
c. heterogeneous mixture.
d. molecule.
Q 2) On heating, solid camphor directly changes to gas, this process is called :
a. evaporation
b. melting
c. deposition
d. sublimation.
Q 3) Proton and neutron is combinely called :
a. electron.
b. atom.
c. nucleon
d. element.
Q 4) Plasma is formed by :
a. ionization of gas.
b. condensation of gas.
c. melting of solid.
d. deposition of gas.
Q 5) ‘He’ indicates :
a. An atom of helium
b. A molecule of helium
c. both a and b
d. An ion of helium.
Answer…. 1- b, 2-d, 3-c, 4-a, 5-c.
References
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
